SAN DIEGO (TheStreet) -- Before I dive into the comparison of InterMune's (ITMN) pirfenidone with Boehringer Ingelheim's nintedanib, Dr. Gary Hunninghake, a specialist in idiopathic pulmonary fibrosis (IPF) at Brigham and Women's Hospital in Boston, says declaring one drug better than the other is a fool's errand.
"You cannot tell from the data published," said Hunninghake, referring to the results from three phase III studies (one from InterMune, two from Boehringer) published Sunday in the New England Journal of Medicine. "Both drugs were evaluated in [IPF] patients with relatively early state disease and both had fundamentally similar outcomes."
I spoke with Hunninghake on Thursday. His editorial on the pirfenidone and nintedanib data, titled "A New Hope for Idiopathic Pulmonary Fibrosis," was also published today in the NEJM. He called the new IPF drugs a game changer and says both are likely to be approved in the U.S. Doctors and their IPF patients here will finally have effective therapies to treat a progressive, lung-scarring disease that has a death rate worse than that of many cancers.
But Dr. Hunninghake, which company -- InterMune or Boehringer -- has the superior IPF drug? "If either company tries to make a claim that their drug is superior over the other, I would say these data do not support it," he says. With all due respect to Dr. Hunninghake, nothing will stop investors from comparing the pirfenidone and nintedanib study results, so let's get at it. In addition to the NEJM publication today, the pirfenidone and nintedanib data are being highlighted at a press briefing sponsored by the American Thoracic Society (ATS). The data will also be presented at the ATS annual meeting on Tuesday. I previewed these data on Friday. Boehringer conducted two phase III studies of nintedanib dubbed "INPULSIS-1" and "INPULSIS-2." The studies enrolled 513 and 548 IPF patients, respectively. The studies' primary endpoint measured the annual rate of decline in forced vital capacity over 52 weeks for patients treated with netedanib and placebo. In INPULSIS-1, the annual rate of decline in FVC was -114.7 ml for nintedanib versus -239.9 ml for placebo -- a relative reduction of 52% and statistically significant. Here's what the result looks like graphically (from the NEJM):
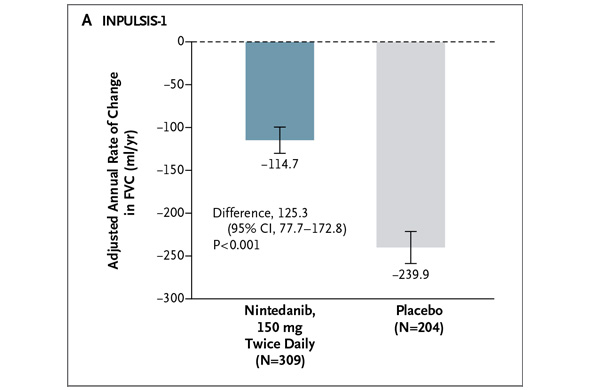 In INPULSIS-2, the annual rate of decline in FVC was -113.6 ml for nintedanib versus -207.3 ml for placebo -- a relative reduction of 45% and statistically significant. Here's what that result looks like graphically (again from the NEJM):
In INPULSIS-2, the annual rate of decline in FVC was -113.6 ml for nintedanib versus -207.3 ml for placebo -- a relative reduction of 45% and statistically significant. Here's what that result looks like graphically (again from the NEJM):
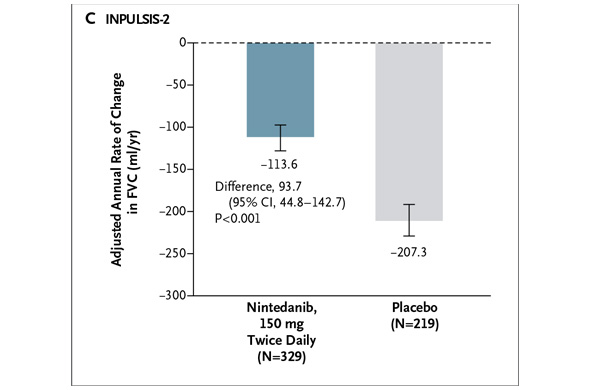 In my preview of the IPF data Friday, I mentioned the primary endpoints of the respective phase III studies designed by InterMune and Boehringer were different. InterMune, however, re-analyzed the pirfenidone study using Boehringer's annual rate of FVC decline endpoint. In InterMune's ASCEND phase III study, the annual rate of decline in FVC was -122 ml in the pirfenidone group and -262 ml in the placebo group -- a relative difference of 53% and statistically significant. Here's what this results looks like graphically:
In my preview of the IPF data Friday, I mentioned the primary endpoints of the respective phase III studies designed by InterMune and Boehringer were different. InterMune, however, re-analyzed the pirfenidone study using Boehringer's annual rate of FVC decline endpoint. In InterMune's ASCEND phase III study, the annual rate of decline in FVC was -122 ml in the pirfenidone group and -262 ml in the placebo group -- a relative difference of 53% and statistically significant. Here's what this results looks like graphically:
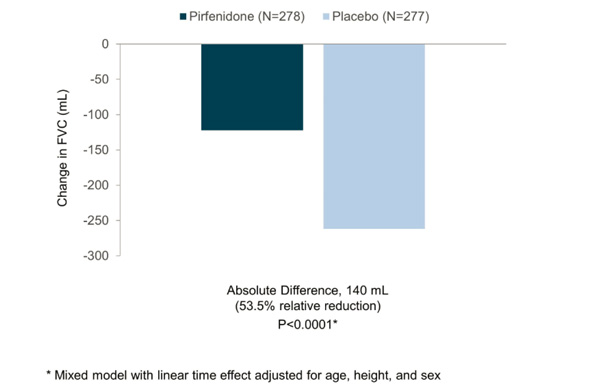 When using Boehringer's primary efficacy endpoint for IPF benefit, InterMune's pirfenidone comes out looking equivalent to nintedanib in one study and superior in the second. [With the caveat that we're comparing across trials.] The primary endpoint of InterMune's ASCEND study measured the proportion of patients in the pirfenidone and placebo arms experiencing a clinically meaningful change (10% or greater) in FVC. The study enrolled 555 IPF patients and achieved its primary endpoint with a high level of statistical significance. At 52 weeks, 16.5% of the IPF patients treated with pirfenidone experienced an FVC decline of 10% or more or death compared to 31.8% in the placebo group. The relative reduction favoring pirfenidone was 48% Boehringer analyzed the nintedanib data from INPULSIS studies to also come up with a categorical description of the drug's efficacy. In INPULSIS-1, 29% of nintedanib-treated patients experienced an FVC decline of 10% or more compared to 43% in the placebo group -- a relative reduction of 33%. In INPULSIS-2, 30% of nintedanib-treated patients experienced an FVC decline of 10% or more compared to 35.5% in the placebo group -- a relative reduction of 15.5%. Using a categorical definition of efficacy in IPF (InterMune's primary endpoint) and comparing across trials, pirfenidone outperformed nintedanib. [Note: InterMune's study enrolled slightly "sicker" IPF patients with a baseline predicted FVC of 67-68% compared to 79-80% in Boehringer's INPULSIS studies.] Let's compare mortality data in the InterMune and Boehringer studies.
When using Boehringer's primary efficacy endpoint for IPF benefit, InterMune's pirfenidone comes out looking equivalent to nintedanib in one study and superior in the second. [With the caveat that we're comparing across trials.] The primary endpoint of InterMune's ASCEND study measured the proportion of patients in the pirfenidone and placebo arms experiencing a clinically meaningful change (10% or greater) in FVC. The study enrolled 555 IPF patients and achieved its primary endpoint with a high level of statistical significance. At 52 weeks, 16.5% of the IPF patients treated with pirfenidone experienced an FVC decline of 10% or more or death compared to 31.8% in the placebo group. The relative reduction favoring pirfenidone was 48% Boehringer analyzed the nintedanib data from INPULSIS studies to also come up with a categorical description of the drug's efficacy. In INPULSIS-1, 29% of nintedanib-treated patients experienced an FVC decline of 10% or more compared to 43% in the placebo group -- a relative reduction of 33%. In INPULSIS-2, 30% of nintedanib-treated patients experienced an FVC decline of 10% or more compared to 35.5% in the placebo group -- a relative reduction of 15.5%. Using a categorical definition of efficacy in IPF (InterMune's primary endpoint) and comparing across trials, pirfenidone outperformed nintedanib. [Note: InterMune's study enrolled slightly "sicker" IPF patients with a baseline predicted FVC of 67-68% compared to 79-80% in Boehringer's INPULSIS studies.] Let's compare mortality data in the InterMune and Boehringer studies.
All-cause mortality in the pooled INPULSIS studies was 5.5% in the nintedanib arms versus 7.8% in the placebo arms -- a 30% relative reduction in the risk of death favoring nintedanib but not statistically significant.
The death rate due to respiratory cause was 3.8% in the nintedanib patients compared to 5% in the placebo groups -- a 26% relative reduction in risk of death but again, not statistically significant.
Switching to InterMune's ASCEND study, the rate of all-cause mortality in pirfenidone-treated patients was 4% versus 7.2% in placebo patients -- a 45% relative reduction in the risk of death but not statistically significant.
With FDA's blessing, InterMune pooled survival data from ASCEND with the previous phase III CAPACITY studies. When these studies are combined, the death rate in the pirfenidone arm is 3.5% compared to 6.7% in placebo-treated patients -- a 48% relative reduction in the risk, which is statistically significant. InterMune can make a legitimate claim to a mortality benefit for pirfenidone in IPF with the pooled data from the ASCEND and CAPACITY trials. How this survival benefit shows up in the pirfenidone label is yet to be determined. Boehringer says the INPULSIS studies were not powered to demonstrate a nintedanib survival benefit. It's interesting however, that the two INPULSIS studies enrolled 1,061 IPF patients, while the combined ASCEND and CAPACITY studies enrolled 1,247 patients. The difference in size isn't all that large. Moving to safety and tolerability: Ten IPF patients treated with nintedanib suffered heart attacks in the two INPULSIS studies (five in each study) compared to two heart attacks reported in placebo-treated patients (one in each study.) Two of the nintedanib heart attacks were fatal; one of the placebo patients also died. The rate of serious adverse events listed as "cardiac disorder" was 5% in the nintedanib arms (pooled) versus 5.4% in the placebo arms (also pooled.) The rate of "serious ischemic disease" reported in the INPULSIS studies was 2.4% in both the nintedanib and placebo arms. There's some new and interesting liver safety data being reported today for the first time from Boehringer's nintedanib studies. Six patients (0.9%) discontinued nintedanib treatment because of elevations in AST, ALT or bilirubin levels -- all markers for liver toxicity. There were no cases of Hy's Law reported in the studies. Eight percent of nintedanib patients experienced elevation of ALT or AST three times the upper limit of normal or greater. The comparable rate of ALT/AST elevations above three times the upper limit of normal for InterMune's pirfenidone was 3%. One case of Hy's Law in a pirfenidone-treated patient was reported. Investors have previously raised some concerns about the liver safety of pirfenidone, but these new nintedanib data suggest Boehringer's drug is no better, perhaps worse. As previously reported, the rate of diarrhea in the nintedanib patients was 61.5% and 63.2% in the two INPULSIS studies. The placebo-adjusted rate of diarrhea was 43% and 45%. However, only 5% of patients discontinued from the two studies due to the diarrhea. Other gastrointestinal-related adverse events of note were 17% and 19% placebo-adjusted rates of nausea. Vomiting was reported in 11% and 7% of patients, adjusted for placebo. Moving to InterMune's study, rash was reported by 28% of pirfenidone-treated patients compared to 8.7% in the placebo arm -- a 19% difference. The placebo-adjusted rate of nausea attributed to pirfenidone was 23%, vomiting 4%. There's a lot to digest here already so I won't go into detail on the key secondary efficacy endpoints of the respective IPF studies except to recount that InterMune's pirfenidone demonstrated a progression-free survival benefit while Boehringer showed a reduction in the time to first acute exacerbation in INPULSIS-2 but not INPULSIS-1. But let's end with some more comparative efficacy data and slides.
The mean decline from baseline in FVC was 235 ml for pirfenidone and 425 ml for placebo -- a relative difference of 45%. Here's what that looks like (From the NEJM):
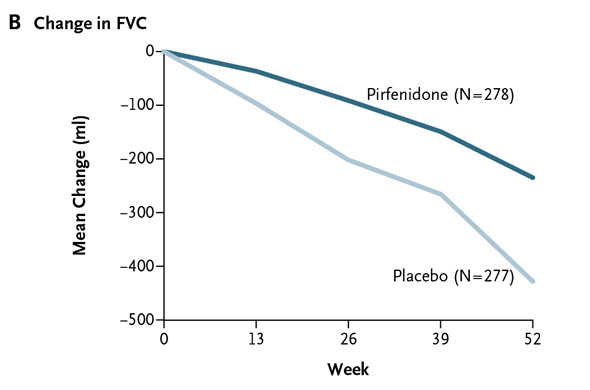
This is what the comparable mean FVC declines look like in Boehringer's two studies:
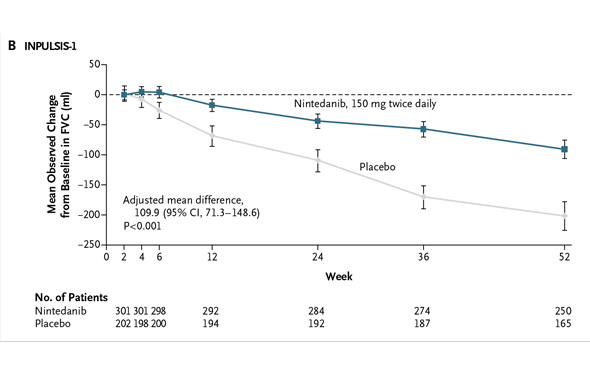
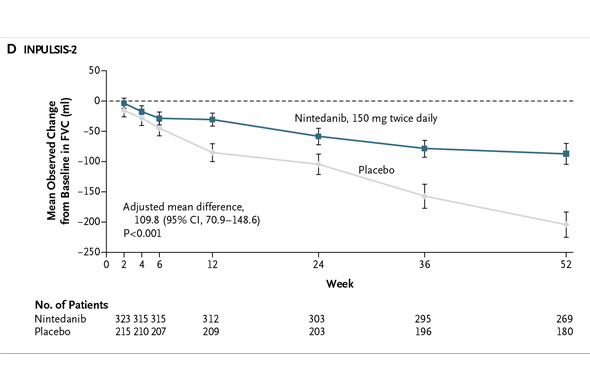
When the Boehringer trials are pooled, the relative difference in FVC decline was 54% favoring nintedanib over placebo. That's superior to pirfenidone.
-- Reported by Adam Feuerstein in Boston
No comments:
Post a Comment